What's All The Fuss About Diamonds?
When you cut through all the hype, is diamond really such a special gemstone?
The answer is a resounding yes!
By Barry B. Kaplan

Diamond's are made of "star
stuff". The earth and its mantle,
where diamonds are formed,
have their origins in a Nebula
like this one, the Carina or
Keyhole Nebula.
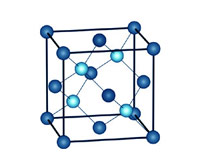
The Diamond Crystal Lattice.

Diamond's high coefficient of
dispersion provides for
a dazlling display of "fire".
Like no other
Diamond is like no other substance. Except for certain trace elements like boron and nitrogen, it is
composed entirely of carbon, the fundamental building block of all life on earth.
The hardest substance known
Carbon is the key ingredient of graphite, a substance that is exceptionally soft. Diamond, on the other
hand, made from the very same core element, is the hardest substance known to man. The dichotomy is resolved
when we understand the crystal structure of carbon atoms in diamond.
A quick high-school chemistry revision may be in order. Neutral carbon atoms consist of six protons (positively
charged particles), and six electrons (negatively charged particles). Four of these electrons, called valence
electrons, are available to bond with neighboring atoms. In diamond, all four electrons bond with adjacent atoms,
forming covalent bonds - the strongest type of chemical bond. Each carbon atom is therefore connected to four
other carbon atoms, creating a rigid and highly symmetrical crystal structure that provides diamond with its
unmatched strength.
Diamond is produced deep within the earth, in an area known as the mantle. Scientific evidence suggests that
the mantle was formed about 4 billion years ago. Thus, diamond may very well consist of the oldest elemental
carbon found on earth.
Repels water and attracts grease
Diamond is the only substance that can scratch another diamond. It is exponentially harder than the next
hardest substance, corundum, of which sapphire and ruby are comprised. Furthermore, diamond repels water
and adheres to wax and grease. It is this remarkable property, that allows diamond to be easily separated
from other minerals in the mining process.
Amazing density
Carbon has a relatively low atomic weight, yet diamond is amazingly dense - 3.51 grams per cubic centimeter.
It took millions of years, a pressure of 55,000 atmospheres and temperatures of 1,400 degrees Celsius to
produce diamond.
Superior brilliance and luster
In addition to the prized characteristics outlined above, diamond is also well known for its superior
brilliance and luster. Like other high density materials, diamond has a high refractive index (ability
to bend/slow light). When passing through diamond, light slows to a leisurely 77,000 miles per second.
Diamond's high refractive index - 2.42 compared to 1.52 in glass - is also a good prognosticator of its
high reflectance (ability to reflect light). Diamond also has a high coefficient of dispersion. When
white light passes through a diamond, it is separated into a rainbow spectrum. When the different
wavelengths of light interact with the diamond, the shorter wavelengths like blue are bent more than
the longer wavelengths like red. This property produces an effect called "fire".
Excellent thermal conductor
Except for some very rare examples, most diamonds are poor electrical conductors. However, diamond
possesses excellent thermal conductivity. When diamond is referred to as ice, it is meant quite literally.
Diamond has four times the thermal conductivity of copper, itself a superb conductor. When diamond is put
to the lips, it draws heat away from the body, making diamond feel cold to the touch.
The most celebrated
Unmatched in hardness, able to repel water and attract grease, of superior density, exhibiting unmatched
brilliance and luster, highly reflective, and thermally conductive, diamond has earned its place as the
most celebrated and extraordinary gemological specimen in nature.



